How do batteries work? Read on to Find Out the Basics!
Without batteries, the world would be a very different place. There would be no cars, no mobile phones, and no computers, to name a few differences.
If you thought about it, you would realize how different your life would be without batteries. Despite our dependence on them, many people don’t know or understand how batteries work.
So, let’s take a look!
First, it is important to remember that energy cannot be stored or created. It can only be converted from one form to another.[1] Batteries do not produce electricity. Instead, they convert stored chemical energy into electrical energy.
It’s a very simple chemical reaction that produces the flow of electricity in a battery. Here, we explain a little about the humble battery. This includes the anatomy of a battery and how batteries work. And we’ll look at the difference between rechargeable and non-rechargeable batteries.
Table of Contents
The History of the Battery
Batteries have been around a lot longer than you probably realize. In 1799, an Italian physicist, Count Alessandro Volta made the first ever battery.[2]
This crude model consisted of alternating layers of metal plates and brine-soaked paper. This was the Voltaic plate. It was the first device to deliver a constant and consistent electrical current.
Volta’s original design has been improved on since. But his name forever lives on in Volts and Voltage, the standard units of electromotive force.
In 1836, English chemist John Frederick Daniell made a battery known as the Daniell cell.[3]
This battery design included metal plates suspended in metal-based solutions. This made it great for powering stationary objects but unsuitable for portable items.
In 1898, the Eveready Battery company produced the first commercial battery.[4] It then went on to produce batteries for Energizer.[5]
What is a battery?
When attempting to answer ‘how do batteries work’, it is important to know what a battery actually is.
A battery is any device that can store electrical energy for use at another time. We use them to power small items such as watches and phones as well as much larger items. These include medical equipment, security lights, and even cars.
Within, a self-contained chemical reaction produces a small amount of electrical energy. This electrical energy is released over days, weeks, months or even years.
A portable power source has always been something that humans have strived for. Even prehistoric man realized the importance of being able to transport fire as a source of heat.
Batteries have provided us with an instant and convenient source of power. And now we can go about our lives with ease.
Anatomy of a battery
The working part inside a battery is the cell. This is contained within a metal and plastic casing to protect us from the chemicals inside.
A battery is usually composed of several cells. Within each of these cells there are three basic parts:
- Two Electrodes,
- An Electrolyte
- A separator.
The two electrodes are made from a conductive material, usually metal. Each has a different function.
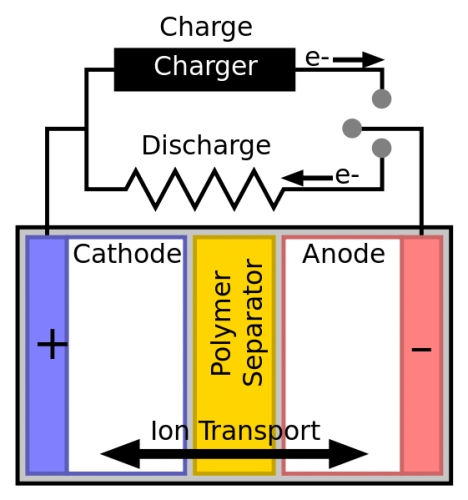
The electrons are repulsed by the Anode(-) but attracted to the Cathode(+). So, they flow from the negative to the positive.
The electrodes bathe in a liquid or gel called an electrolyte. The electrolyte contains positively charged particles, known as ions.
These are created at the same time as the Anode produces negatively charged electrons. The ions flow through the separator toward the Cathode and balance the reaction.
The arrival of positively charged ions at the cathode balances the negatively charged electrons that are arriving through the external wire.
As you can see in the diagram, the separator separates the two electrodes. This is a non-conductive material such as plastic or paper. It prevents the electrodes from touching and causing a short circuit.
How do Batteries work?
OK, so how do batteries work?
The Anode described above reacts with the free ions in the electrolyte. This releases electrons into the electrolyte in a chemical process called oxidation.
On the Cathode, a different chemical reaction known as reduction occurs. This makes the electrode accept electrons from the electrolyte.
Combined, this electrochemical reaction is an oxidation-reduction reaction or Redox reaction.
This flow of electrons from one electrode to another is what we know as electrical current. Yet, inside the battery, the separator is not conducive. It PREVENTS the flow between the anode and cathode.
But the electrons WANT to move from the negative electrode to the positive.
As the separator prevents this, they need another path from the anode to the cathode. We create this when we attach a device to the battery.
See the diagram. When you connect something to a battery, you CREATE a path between the positive and negative terminals.
The electrons can now find a path THROUGH the electrical item.
For example, when you connect a bulb to a battery, electrons flow into the bulb. They then travel through the filament, which becomes hot enough to glow. Then they flow out of the bulb and back into the battery, completing the circuit.
Rechargeable Versus Non-rechargeable
The difference between rechargeables and non-rechargeable is the reversibility of the electrons. If they are not reversible, the battery is not rechargeable.
Let me put it another way. In a non-rechargeable battery, the anode will release electrons until there are no free electrons left. This means the battery can no longer produce a flow of electrons. The battery is dead. This process is irreversible.
In rechargeable batteries, this reverses when the battery attaches to a charger. Attaching a charger reverses the flow of electrons. The anode replenishes with electrons.
Rechargeable batteries do eventually lose their ability to produce an electric current. But only after hundreds of recharge cycles.
Like reading How do batteries work? Check out are Related Articles


