How to Use a Battery Hydrometer to Test Your Battery’s Health. Learn how to measure the specific gravity of the electrolyte and avoid costly battery failures.
If you own a car, you know how important it is to keep your battery in good shape. If your battery is weak or dead, you can get stuck on the side of the road. It can also harm your engine or electrical system. But how do you know if your lead acid battery is healthy or not?
The answer is you use a battery hydrometer! This device uses specific gravity to measure battery charge. You can use a battery hydrometer to test the state of charge in each cell of your battery. The higher the specific gravity, the higher the charge. The lower the specific gravity, the lower the charge.
Sounds easy, right? Well, not so fast. To use a battery hydrometer, you need to know how to prepare it. In this article, I will explain how to use it safely and adjust readings for different conditions.
At first, using a battery hydrometer may seem scary, but it’s actually easy with practice. Before getting started, it’s essential to understand what a hydrometer does. To get accurate results and stay safe, it’s important to use the device correctly.
Let’s do this!
Table of Contents
Key Takeaways
- Battery hydrometers measure the liquid’s density. This shows how much power a lead-acid battery has.
- Proper preparation and technique ensure accurate, safe usage of a hydrometer.
- To make the device last longer and give accurate results, take care of it and avoid common errors.
How to Use a Battery Hydrometer – What is a Battery Hydrometer?
Before we get waist deep into the creek of how to use your hydrometer, let’s think about what it actually is.
Well, it’s a handy tool that helps us check the health of our lead-acid batteries. It works by measuring the specific gravity of the battery’s electrolyte. This value gives us important information about the battery’s condition. So, let’s start by understanding what specific gravity is.
Your hydrometer works by comparing the density of the electrolyte to the density of water.
The specific gravity of pure water is 1.000. The uncharged battery fluid is a sulphuric acid solution with a specific gravity of 1.120. Charging the battery releases electrolytes into the solution, raising the specific gravity to a maximum of 1.265 when fully charged. The density of the bulb of the hydrometer allows its floatation level to measure the specific gravity of the battery acid and hence the level of charge.
This reading shows how much the battery is charged or discharged. If the specific gravity is higher, the battery is fully charged. If it’s a bit on the low side, the battery is undercharged or failing.
Using a battery hydrometer is quite straightforward. First, you extract a sample of electrolyte from each cell in the battery. Then, you check the specific gravity using the hydrometer. Measuring each cell separately helps to detect imbalances or potential issues.
Preparations
My old nan always used to say to me, “Steve, safety is important”. Not catchy, I know, but certainly apposite. And it applies more to batteries than most things.
Always wear protective gear, like gloves and safety goggles. Remember, the electrolyte contains sulfuric acid, so handle it with care to avoid injury or ruining your threads.
Next, we need to check the battery’s condition. Make sure there are no leaks, cracks, or bulges. Clean the battery to remove any dirt or debris. A soft brush and a mix of baking soda and water are ideal for this step.
Before testing, check the electrolyte levels in the battery cells. If it is low, top up the cells with distilled water. To ensure an accurate reading, we need to make sure the battery is fully charged.
While using the hydrometer, we need to keep it nice and clean. Any contamination could affect our results. So, clean the device before and after taking readings.
Last, make sure to take the temperature of the electrolyte into account. Temperature affects the electrolyte’s density, which in turn affects the hydrometer reading.
Step-by-Step Guide to Using a Battery Hydrometer
Now, down to business.
Taking a Hydrometer Reading
To take a hydrometer reading, draw the electrolyte into the hydrometer several times. It is like sucking up liquid with a pipette. Reminds me of chemistry classes.
This action allows the float to float freely. It also allows the built-in thermometer to adjust to the electrolyte temperature. Hold the hydrometer vertically at eye level, ensuring the float floats freely.
Interpreting the Results
Now, let’s interpret our reading. Note the number on the scale where the electrolyte meets the float. This number represents the specific gravity of the electrolyte in the battery.
A higher specific gravity indicates a higher charge state. A fully charged battery should have a specific gravity between 1.265 and 1.299.
Here’s a table of all of the specific gravities and what they mean in terms of state of charge.
| Specific Gravity (sg) | State Of Charge (%) |
|---|---|
| 1.265 | 100 |
| 1.225 | 80 |
| 1.190 | 60 |
| 1.155 | 40 |
| 1.120 | 20 |
| 1.080 | 0 |
Temperature Compensation
So we test our electrolytes and we have our reading. Easy peasy, right?
Wrong. There’s a catch.
Remember when I said that the hydrometer tests the specific gravity of the electrolyte? Well, this reading is also affected by the temperature.
The electrolyte solution in a lead-acid battery expands when warm and contracts when cold. This affects the density and specific gravity of the electrolyte.
Hydrometers measure the specific gravity of the electrolyte to determine the state of charge. And changes in temperature can alter our results.
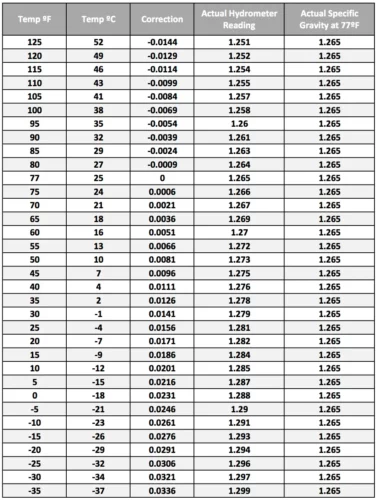
For example, if the electrolyte is cold, it may read 1.250 on the hydrometer. But once adjusted for the temperature, the actual specific gravity maybe 1.275. Without compensating, you might think the battery charge is lower than it really is.
Here’s a general guideline for temperature correction in lead-acid batteries:*
- Below 25°C (77°F), add points (0.003 per 10°F or 0.0017 per 5°C)
- Above 25°C (77°F), subtract points (0.003 per 10°F or 0.0017 per 5°C)
When using a manual hydrometer, we need to refer to a conversion chart to find the temperature-adjusted gravity. One a little like this one:
We also need to know the temperature. The weather app on your phone is fine for that if you don’t have a thermometer.
If this all sounds like too much hassle, digital models do this automatically for more precise measurements. Most modern battery hydrometers have built-in thermometers and automatic temperature compensation to adjust for this effect.
This allows them to display an accurate, temperature-corrected specific gravity reading.
*Please note that these are approximate values and the actual correction factor can vary depending on the specific gravity of the electrolyte and the calibration temperature of your hydrometer. Always refer to the manufacturer’s instructions for the most accurate information.
Should I Remove the Battery
I often get asked whether you should remove your battery to conduct a hydrometer test. The answer is: it’s up to you!
However, there are advantages to removing the battery entirely. So I always do.
Enhancing Safety: When working around a mounted battery, the potential for acid burns or electrical shock goes up. Removing the battery provides a safer environment for cleaning, accessing cells, and conducting tests.
Improved Accessibility: In-vehicle batteries often pose challenges with limited visibility and awkward physical access to battery caps and cell openings. This makes testing more difficult.
Enhanced Cleanliness: Testing a clean battery yields better results. Removing the battery makes it easier to wash and remove any residue or grime buildup thoroughly.
Greater Control: With the battery removed from the vehicle, you gain more control over its positioning and stability. This means easier visual alignment and hydrometer insertion and prevents tipping or spilling.
Better Portability: An unmounted battery allows for easy transportation to a charging station or load tester for any necessary follow-up tests.
Mistakes to Avoid When Using a Battery Hydrometer
So now you have a good idea of how to test your Specific Gravity. Simple, right?
Well, possibly not. Believe it or not, I have seem plenty of people come a cropper. That’s why I wrote this list of things to avoid when hydrometer-ing:
- Not charging the battery before testing is a big mistake. It’s important to charge the battery to get accurate readings.
- Wearing proper safety gear. Battery hydrometers involve handling sulfuric acid, which is corrosive. Wear goggles, gloves, closed-toe shoes, and a rubber apron.
- Not cleaning the battery before testing can also lead to inaccurate results. A clean hydrometer can access the electrolyte correctly for precise measurements.
- Using a battery hydrometer on maintenance-free batteries. This is incorrect and can damage your battery or hydrometer. Battery hydrometers are only suitable for lead-acid batteries with removable caps.
- Read the hydrometer results correctly. Incorrect readings on the hydrometer can lead to wrong battery analysis. To keep a battery in shape, you need to know what specific gravity readings mean for the battery’s charge (see table!)
- Finally, avoid over-squeezing the hydrometer when drawing in the battery fluid. This can cause the hydrometer’s float to stick, leading to inaccurate results. Squeeze the bulb gently to get a correct reading.
Gel Cell and AGM Batteries
Now, if you have an AGM or gel cell battery, you may not want to be left out of this party. Well, I’ve got some good news for you: you can buy hydrometers designed for those battery types.
The core specific gravity measurement and interpretations are similar. But the types of electrolytes and hydrometer equipment vary across the different types of lead-acid batteries.
AGM Batteries
AGM batteries are a unique breed. Their electrolyte is absorbed into fiberglass mats instead of being in liquid form like traditional flooded batteries. So when it comes to testing these batteries, you’ll need an AGM hydrometer with a longer testing stem to reach the electrolyte through the mats.
Another perk of AGM batteries is that they don’t require fluid level checks or refills with water, making maintenance a breeze. Plus, thanks to their design, AGM batteries usually need less frequent testing than their flooded brothers.
Gel Cell Batteries
Gel cell batteries are a fascinating bunch, housing their electrolyte in a gel-like consistency. When it comes to testing these batteries, we need special gel-cell hydrometers to measure the viscosity and thickness of the electrolyte.
But a word of warning, gel cell batteries shouldn’t be tested too frequently, as this can potentially damage their cell plates. After the test, they need a total recharge before being put back into service.
Before You Go…
You have just learned how to use a battery hydrometer to measure the health of your car battery. But what if your battery is too old or damaged to be recharged? Do you know what to do with it? If you think you can toss it in the garbage, think again. You need to read my next article: “How to Properly Dispose of a Car Battery”.
I will explain why throwing away your car battery is a bad idea. Follow these steps to handle your battery safely and legally. You see, a car battery is not like any other battery. They contain things like lead, acid, and hydrogen, which can cause big problems for you and the Earth. So don’t risk it, and read this article to learn how to dispose of your car battery the smart way.
Frequently Asked Questions
Here’s the FAQs.
What should a fully charged battery’s hydrometer reading be?
A fully charged battery’s hydrometer reading should be between 1.265 and 1.299. This indicates the battery is operating at optimal capacity. Lower readings may signal an undercharged or failing battery.
How do you test battery gravity with a hydrometer?
To test battery gravity with a hydrometer, you’ll need to:
- Disconnect and remove the battery.
- Clean the battery using a baking soda paste.
- Open the battery cells.
- Carefully insert the hydrometer into each cell.
- Record the specific gravity reading of the electrolyte solution.
- Compare the readings to the healthy range (1.265-1.299).
How accurate are battery hydrometers?
Battery hydrometers are generally accurate and reliable tools for measuring specific gravity. But, their precision may vary from one model to another. A good hydrometer gives accurate results to keep your battery healthy.
What is the price range for battery hydrometer testers?
Battery hydrometer testers can cost anywhere from $10 to $50. I recommend doing some research to find a unit that best suits your needs and budget.
How can I find the best hydrometer for battery testing?
To find the best hydrometer for battery testing, consider the following factors:
- Rating and reviews from other users
- Accuracy and precision of measurements
- Ease of use and built-in safety features
Don’t forget to compare prices and gauge the overall of the product.
Are there digital battery hydrometers available?
Yes, digital battery hydrometers are here! They have digital displays, easy-to-read results, and increased accuracy. If you need a modern and accurate way to test batteries, you should consider a digital hydrometer.
Related Posts
- LiFePo4 vs Lead Acid Batteries: We Compare 7 Key Attributes in The Ultimate Guide to Choosing the Best Battery
- How do Batteries Work – Find out Here in our Helpful Guide
- How to Maximize Battery Potential: Discover the 7 Crucial Charge Types you NEED to know!
- What Is An AGM Battery? – Discover everything you NEED to know in our Helpful Guide!